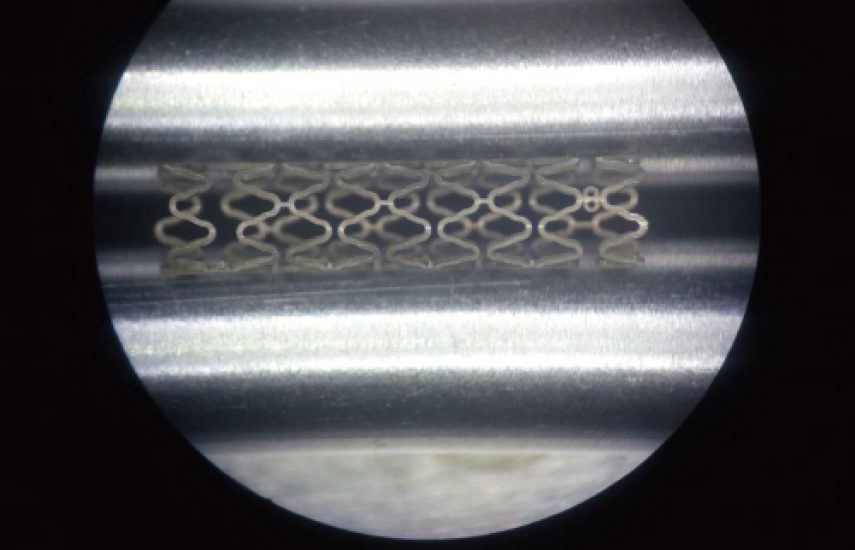
סטנט מסגסוגת מגנזיום 3.2 מ"מ
3.2 mm Magnesium Alloy Stent: biodegradable vascular support device
The 3.2 mm Magnesium Alloy Stent is an innovative biodegradable vascular stent designed for the treatment of coronary artery disease. Crafted from a high-performance magnesium alloy, this stent provides temporary scaffolding to maintain vessel patency during the critical healing period, after which it gradually degrades, reducing the risk of long-term complications associated with permanent implants. Its 3.2 mm diameter ensures compatibility with a wide range of vessel sizes, making it a versatile solution for interventional cardiology.
תכונות עיקריות:
- Biodegradability: Naturally degrades over time, eliminating the need for secondary removal and reducing long-term risks.
- ביולוגיות תאימות: Minimizes adverse tissue reactions, promoting healing and integration.
- Mechanical Strength: Offers sufficient radial force to support the vessel during the initial post-implantation phase.
- Controlled Degradation Rate: Engineered to ensure structural integrity until the vessel heals, then safely dissolves.
- Radiopacity: Enhanced visibility under imaging, aiding precise placement and monitoring.
Certification and Standards:
- CE Marking: Complies with European Union regulations for medical devices, ensuring safety and efficacy.
- ISO 13485: Certified under international quality management standards for medical device manufacturing.
- FDA Clearance: Pending or achieved, depending on market requirements, for use in the United States.
- Biocompatibility Testing: Meets ISO 10993 standards for biocompatibility, confirming safety for human use.
The magnesium alloy used in the 3.2 mm Stent is characterized by:
-
- Lightweight: One of the lightest structural metals, reducing the implant's overall mass.
- High Strength-to-Weight Ratio: Maintains robust mechanical properties despite its light weight.
- Predictable Degradation: Composition is optimized to control the degradation rate, ensuring temporary support followed by safe absorption.
- Non-Toxic Byproducts: Degradation products are naturally occurring and safely metabolized by the body.
- Elastic Modulus: Matches closely with human vascular tissue, minimizing stress shielding and promoting better integration.
This combination of features and certifications makes the 3.2 mm Magnesium Alloy Stent a state-of-the-art choice for modern cardiovascular interventions, offering a balance of efficacy, safety, and patient-centric design.
This stent is primarily used in percutaneous coronary interventions (PCI) for patients with coronary artery stenosis. It is particularly suitable for individuals who may benefit from a temporary scaffold that degrades over time, such as those at risk of in-stent restenosis or late stent thrombosis. The 3.2 mm diameter makes it ideal for medium-sized vessels, providing effective support while minimizing the risk of over-expansion or vessel damage.
- Diameter: 3.2 mm
- Length Options: Available in 12 mm, 18 mm, and 24 mm to accommodate various lesion lengths.
- Material: High-purity magnesium alloy with proprietary alloying elements for enhanced performance.
- Deployment: Balloon-expandable, ensuring controlled and precise expansion.
- Degradation Period: Approximately 6-12 months, tailored to the healing timeline.
- Compatibility: Designed for use with standard catheterization and imaging systems.