Biocompatibility of Nitinol Tubes: ISO 10993 Standards and FDA Requirements for Medical Implants
TL;DR — Key Takeaways in 30 Seconds
Nitinol biocompatibility depends on surface treatment that forms a protective TiO₂ oxide layer, preventing nickel ion release. Despite containing 50-51% nickel, properly processed nitinol meets all ISO 10993 requirements and has proven clinical safety across millions of implanted devices.
Critical Parameters:
- Surface oxide layer: TiO₂, 3-20 nm thick
- Nickel release rate: <0.2 μg/cm²/week (after electropolishing)
- Surface roughness: Ra <0.3 μm for blood-contacting devices
- Required testing: cytotoxicity, sensitization, implantation, hemocompatibility
Why Biocompatibility Matters for Nitinol
Nitinol tubes are widely used in medical implants due to their unique shape memory effect and superelasticity. However, the high nickel content (50-51 atomic %) raises concerns about biocompatibility — the material’s ability to perform safely within the human body.
This article explains how proper manufacturing transforms nitinol into a biocompatible material meeting stringent regulatory requirements.
The Nickel Paradox: High Content, Low Release
Nickel Content Comparison
| Material | Nickel Content | Biocompatibilidad: |
|---|---|---|
| Nitinol (NiTi) | 50-51 at.% | Approved for implants |
| Acero inoxidable 316L | 10-14 wt.% | Gold standard |
| L605 Cobalt-Chromium | 9-11 wt.% | Excellent |
| MP35N | 33-37 wt.% | Excellent |
Nickel is a known allergen affecting 10-15% of the population. How can a material with 50% nickel be biocompatible?
The Protective TiO₂ Oxide Layer
When properly treated, nitinol forms a stable titanium dioxide (TiO₂) layer that acts as a barrier between the nickel-rich bulk material and biological tissues:
| Property | Value | Significance |
|---|---|---|
| Composition | Primarily TiO₂ | Same as pure titanium surface |
| Thickness | 3-20 nm | Sufficient barrier function |
| Structure | Amorphous/anatase | Chemically stable |
| Self-healing | Yes | Reforms if damaged |
| Stability | Inert in physiological pH | Long-term durability |
This TiO₂ layer is the same oxide that makes pure titanium highly biocompatible — essentially creating a titanium-like surface on nitinol.
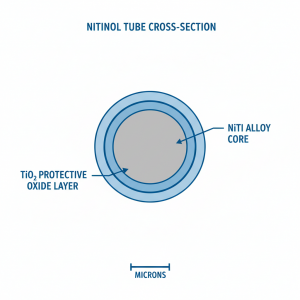
Surface Treatment Methods for Biocompatibility
1. Electropolishing (Primary Method)
Electropolishing is the most critical step for achieving nitinol biocompatibility. This electrochemical process removes the damaged surface layer created during corte por láser and promotes TiO₂ formation.
Process Parameters:
| Parameter | Typical Value | Effect on Biocompatibility |
|---|---|---|
| Electrolyte | H₂SO₄ + HCl | Selective nickel dissolution |
| Temperature | 60-80°C | Controlled reaction rate |
| Current density | 0.5-2.0 A/cm² | Surface smoothness |
| Duration | 1-10 minutes | Material removal depth |
| Voltage | 10-30 V | Oxide layer quality |
Results After Electropolishing:
- Surface roughness: Ra <0.3 μm (from Ra 1-2 μm after laser cutting)
- Nickel surface content: Reduced from 50% to 5-15%
- TiO₂ layer: 5-10 nm, uniform coverage
- Recast layer: Completely removed
2. Chemical Passivation
Acid treatment enhances the protective oxide layer:
- HNO₃ passivation (ASTM F86): Standard method, 20-40% HNO₃ at 50-60°C for 30-60 minutes
- Citric acid: Gentler alternative, environmentally friendly
3. Thermal Oxidation
Controlled heating creates thicker TiO₂ layer:
- Temperature: 400-600°C
- Duration: 10-60 minutes
- Result: TiO₂ layer 50-200 nm thick
Important: Thermal oxidation must be performed after shape setting to avoid altering transformation temperatures.
ISO 10993: Biological Evaluation Requirements
Overview of Applicable Standards
| ISO 10993 Part | Title | Application to Nitinol |
|---|---|---|
| Part 1 | Evaluation framework | Test selection guidance |
| Part 4 | Hemocompatibility | Cardiovascular stents |
| Part 5 | Cytotoxicity | All devices |
| Part 6 | Implantation | Permanent implants |
| Part 10 | Sensitization | Nickel allergy assessment |
| Part 11 | Systemic toxicity | Metal ion release effects |
| Part 12 | Sample preparation | Testing methodology |
| Part 15 | Degradation products | Corrosion analysis |
Cytotoxicity Testing (ISO 10993-5)
Purpose: Evaluate whether material extracts cause cell death.
Methods:
- Direct contact: Cells grown on material surface
- Extract testing: Cells exposed to 72h extracts at 37°C
- Agar diffusion: Indirect contact assessment
Acceptance Criteria: Cell viability >70% vs. negative control
Nitinol Performance: Properly surface-treated nitinol consistently achieves >90% cell viability.
Sensitization Testing (ISO 10993-10)
Due to high nickel content, sensitization testing is critically important for nitinol.
Methods:
- Guinea Pig Maximization Test (GPMT): Most sensitive
- Local Lymph Node Assay (LLNA): Mouse model alternative
Nitinol Performance with Proper Electropolishing:
- Sensitization rate in GPMT: <5%
- Clinical allergic reactions: <0.1% in post-market surveillance
Implantation Testing (ISO 10993-6)
Protocol:
- Animal model: Rabbit or rat
- Sites: Subcutaneous or intramuscular
- Duration: Short-term (1-4 weeks), long-term (12-52 weeks)
Evaluation Parameters:
- Fibrous capsule thickness
- Inflammatory cell infiltration
- Necrosis presence
- Neovascularization
Nitinol Results: Thin fibrous capsule (<100 μm), no chronic inflammation at 52 weeks — comparable to titanium controls.
Hemocompatibility (ISO 10993-4)
For cardiovascular stents and blood-contacting devices:
| Category | Tests | Purpose |
|---|---|---|
| Thrombosis | In vivo models | Clot formation assessment |
| Coagulation | PT, aPTT, fibrinogen | Clotting cascade activation |
| Platelets | Count, activation markers | Adhesion and aggregation |
| Complement | C3a, C5a, SC5b-9 | Immune system activation |
Surface Roughness Impact on Thrombogenicity:
| Surface Finish | Ra Value | Risk Level |
|---|---|---|
| As-cut (laser) | 1-3 μm | High |
| Mechanical polish | 0.5-1 μm | Moderate |
| Electropolished | <0.3 μm | Low |
| Mirror finish | <0.1 μm | Minimal |
Nickel Ion Release: Quantification and Limits
Testing Methodology (ISO 10993-12, -15)
Static Immersion Protocol:
- Medium: PBS or Ringer’s solution
- Temperature: 37°C ± 1°C
- Duration: 1, 7, 14, 30, 60, 90 days
- Analysis: ICP-MS or ICP-OES
- Reporting: μg/cm²/week
Nickel Release by Surface Condition
| Surface Treatment | Ni Release (μg/cm²/week) | Regulatory Status |
|---|---|---|
| As-cut (laser) | 5-50 | Unacceptable |
| Mechanical polish | 1-5 | Borderline |
| Electropolished | 0.1-0.5 | Acceptable |
| Electropolished + passivated | <0.2 | Optimal |
Safety Margin Calculation
For typical coronary stent (surface area ~2 cm²):
- Tolerable daily intake (TDI) for nickel: ~35 μg/day (70 kg adult)
- Maximum acceptable release from device: <0.5 μg/day
- Achieved with electropolishing: 0.02-0.1 μg/day
- Safety margin: 5-25x below threshold
FDA Regulatory Pathways
Device Classification
| Device Type | FDA Class | Pathway | Examples |
|---|---|---|---|
| Peripheral stents | Class II | 510(k) | Iliac, femoral, carotid |
| Guidewires | Class II | 510(k) | Cardiovascular access |
| IVC filters | Class II | 510(k) | Vena cava filters |
| Coronary DES | Class III | PMA | Drug-eluting stents |
| TAVR frames | Class III | PMA | Valve stents |
510(k) Biocompatibility Requirements
- Material characterization: Composition per ASTM F2063
- Surface analysis: XPS, SEM, profilometry
- ISO 10993 testing: Based on contact type and duration
- Comparison: Substantial equivalence to predicate device
- Corrosion testing: ASTM F2129 (Epit >+300 mV vs. SCE)
Key ASTM Standards
| Standard | Title | Requirement |
|---|---|---|
| ASTM F2063 | Nitinol composition | Ni 54.5-57.0 wt.%, impurities controlled |
| ASTM F2129 | Corrosion testing | Epit, icorr measurement |
| ASTM F2082 | Transformation temperature | DSC method for Ms, Mf, As, Af |
Quality Control for Biocompatibility
Incoming Material Inspection
| Test | Method | Acceptance |
|---|---|---|
| Composition | ICP-OES | Per ASTM F2063 |
| Transformation temp | DSC | Af <35°C (typical) |
| Inclusions | Metallography | <10 μm, <0.05% area |
Post-Electropolishing Verification
| Parameter | Method | Acceptance Criteria |
|---|---|---|
| Surface roughness | Profilometry | Ra <0.3 μm |
| Surface composition | XPS | TiO₂ dominant, Ni <15% |
| Visual inspection | Microscopy | Mirror finish, no pitting |
| Corrosion resistance | ASTM F2129 | Epit >+300 mV vs. SCE |
Batch Release Testing
- Cytotoxicity: Every lot or skip-lot (based on validation)
- Nickel release: Periodic verification
- Surface analysis: Per sampling plan
Clinical Evidence: Long-Term Safety
Published Data Summary
Peripheral Vascular Stents:
- Registry data: >100,000 patients
- Allergic reaction rate: <0.1%
- Nickel sensitivity correlation: No increase
TAVR Valve Frames:
- Clinical trials: >50,000 patients
- Nickel-related complications: None reported
Patients with Known Nickel Allergy:
Current evidence indicates:
- Cutaneous nickel allergy ≠ systemic reaction to properly passivated implants
- Most nickel-sensitive patients can safely receive nitinol devices
- Preoperative patch testing recommended for highly sensitized individuals
FAQ — Frequently Asked Questions
1. Is nitinol safe for patients with nickel allergy?
For most patients with cutaneous nickel allergy (contact dermatitis from jewelry), properly electropolished nitinol implants are safe. The TiO₂ surface layer prevents nickel-tissue contact. Clinical studies show no increased adverse events in nickel-sensitive patients.
2. How long does the protective oxide layer last?
The TiO₂ layer is thermodynamically stable and self-healing. Long-term implant retrievals (10+ years) show intact oxide layers with no significant degradation.
3. Does electropolishing change shape memory properties?
No. Electropolishing removes only 5-20 μm of surface material. The shape memory effect and transformation temperatures remain unchanged.
4. What surface roughness is required for cardiovascular devices?
Ra <0.3 μm is recommended for blood-contacting devices to minimize platelet adhesion and thrombosis risk. For non-blood-contacting implants, Ra <0.8 μm is typically acceptable.
5. Which sterilization methods are compatible with nitinol?
- EtO (Ethylene Oxide): Preferred, no effect on properties
- Gamma radiation: Up to 50 kGy acceptable
- E-beam: Similar to gamma
- Steam autoclave: Not recommended (oxidation risk)
Conclusion
Biocompatibility of tubos de nitinol depends critically on proper surface treatment. Despite 50-51% nickel content, electropolished nitinol forms a protective TiO₂ layer that:
- Reduces nickel ion release to <0.2 μg/cm²/week
- Passes all ISO 10993 biocompatibility tests
- Demonstrates excellent long-term clinical safety
- Enables unique shape memory properties unavailable in other materials
Key Success Factors:
- Electropolishing: Ra <0.3 μm with verified TiO₂ formation
- Quality Control: Consistent processes, documented validation
- Testing: Complete ISO 10993 battery for device classification
- Documentation: Comprehensive technical file for regulatory submission
Related Resources
Materiales
Technical Articles
- Shape Memory Effect in Nitinol
- Nitinol vs Stainless Steel vs Cobalt-Chromium
- Thin-Wall vs Thick-Wall Nitinol Tubes
