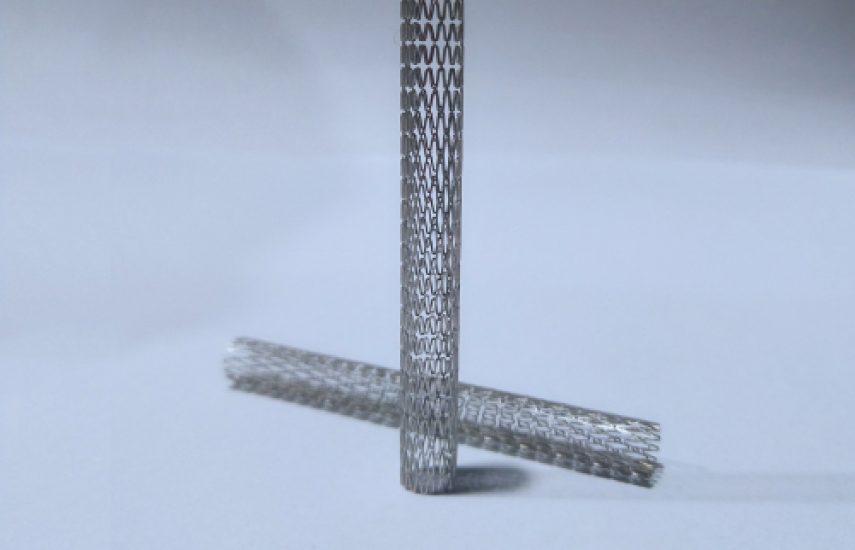
6.5mm Magnesium Alloy Filter
6.5mm Magnesium Alloy Filter – innovative bioreabsorbable protection for vascular health
The 6.5mm Magnesium Alloy Filter represents a groundbreaking advancement in temporary vascular protection, designed to prevent embolic events during interventional procedures. Utilizing a novel bioreabsorbable magnesium alloy, this filter offers effective mechanical filtration while eliminating the need for a second retrieval procedure. Its unique design ensures optimal wall apposition and minimal flow disturbance, providing a safe and transient solution for patient care.
Key Features:
- Bioreabsorbable Material: Naturally dissolves within the body over time, eliminating permanent implant risks.
- Excellent Biocompatibility: Ensures minimal inflammatory response and promotes tissue healing.
- High Filtration Efficacy: Effectively captures embolic debris to prevent downstream complications.
- Optimal Radial Force: Maintains vessel patency without over-expansion.
- Enhanced Radiopacity: Allows for clear visualization during placement and monitoring.
Certification and Standards:
Our 6.5mm Magnesium Alloy Filter is manufactured under stringent quality control protocols, adhering to the highest international medical device standards, including ISO 13485:2016 and CE marking requirements. Each device undergoes comprehensive testing to guarantee its safety, performance, and efficacy in clinical applications.The magnesium alloy used in the 3.2 mm Stent is characterized by:
-
- Lightweight: One of the lightest structural metals, reducing the implant's overall mass.
- High Strength-to-Weight Ratio: Maintains robust mechanical properties despite its light weight.
- Predictable Degradation: Composition is optimized to control the degradation rate, ensuring temporary support followed by safe absorption.
- Non-Toxic Byproducts: Degradation products are naturally occurring and safely metabolized by the body.
- Elastic Modulus: Matches closely with human vascular tissue, minimizing stress shielding and promoting better integration.
This combination of features and certifications makes the 3.2 mm Magnesium Alloy Stent a state-of-the-art choice for modern cardiovascular interventions, offering a balance of efficacy, safety, and patient-centric design.
The 6.5mm Magnesium Alloy Filter is indicated for temporary embolic protection in a range of cardiovascular and peripheral interventional procedures, including but not limited to:
- Carotid artery stenting
- Saphenous vein graft intervention
- Peripheral artery interventions (e.g., in the lower extremities)
- Other procedures where there is a risk of distal embolization
Its temporary nature and reabsorbable properties make it an ideal choice for patients who would benefit from transient protection without the need for additional procedures to remove a permanent device.
- Diameter: 6.5 mm
- Material: Bioreabsorbable Magnesium Alloy
- Working Lengths: Available in various standard lengths
- Pore Size: Optimized for effective embolic capture
- Degradation Time: Controlled reabsorption over a specified period (e.g., 6-12 months, based on specific alloy composition and design)
- Sterilization: EO (Ethylene Oxide) Sterilized
- Packaging: Individually sterile-packed
- Compatibility: Compatible with standard delivery systems and guide catheters