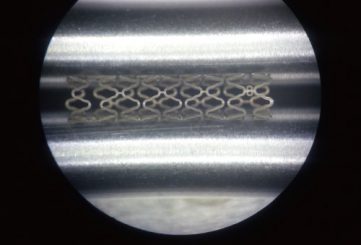
Polylactic Acid Degradable Stent
The Polylactic Acid (PLA) Degradable Stent – a cutting-edge bioabsorbable vascular stent
It is designed to treat coronary and peripheral artery diseases. Unlike traditional metallic stents, this innovative device provides temporary mechanical support to the artery during the healing process and gradually degrades into non-toxic byproducts, eliminating the need for permanent implants. Made from polylactic acid, a biocompatible and biodegradable polymer, the stent supports vessel patency, promotes natural healing, and reduces long-term complications such as restenosis and thrombosis. This environmentally conscious solution aligns with the body’s natural processes, offering a safer and more sustainable approach to cardiovascular interventions.
Key Features:
- Biodegradability: Fully degrades into harmless lactic acid, absorbed by the body over 18–24 months.
- Biocompatibility: Minimizes inflammatory responses and supports natural tissue regeneration.
- Radial Strength: Provides sufficient mechanical support during vascular remodeling.
- Flexibility: Adapts to arterial curvature, reducing stress on vessel walls.
- Reduced Thrombosis Risk: Eliminates long-term foreign body presence, lowering the risk of late stent thrombosis.
- Customizable Design: Compatible with advanced manufacturing techniques like 3D printing for patient-specific solutions.
Certification and Standards:
- FDA-approved for bioabsorbable stent applications (based on precedents like Abbott BVS).
- Complies with ISO 10993 for biocompatibility testing.
- Meets ASTM F3067-14 standards for radial strength and mechanical performance.
- Adheres to ISO 13485 for medical device quality management systems.
Polylactic Acid (PLA): A biodegradable thermoplastic derived from renewable resources like corn or sugarcane.
- Controlled Degradation: Breaks down into lactic acid, metabolized into water and carbon dioxide via the tricarboxylic acid cycle.
- High Biocompatibility: Reduces chronic inflammation compared to metallic stents, promoting endothelialization.
- Tunable Properties: Molecular weight and crystallinity can be adjusted to optimize degradation rate and mechanical strength.
- Eco-Friendly: Derived from sustainable sources, minimizing environmental impact during production and degradation.
- Coronary Artery Disease: Used to restore blood flow in blocked coronary arteries, supporting vessel walls during healing.
- Peripheral Artery Disease: Applicable in peripheral vessels to treat occlusions and maintain patency.
- Pediatric Interventions: Suitable for children due to its degradable nature, avoiding complications from permanent implants.
- Arteriovenous Fistula (AVF) Maturation: Assists in dilating veins for hemodialysis access, degrading after maturation.
- Ureteral and Biliary Support: Potential use in non-vascular systems for temporary support in ureteral or biliary obstructions.
- Material: Poly-L-lactic acid (PLLA) or PLA-based copolymers.
- Diameter: 2.5–6.5 mm (customizable for specific vessels).
- Strut Thickness: 100–150 µm, optimized for radial strength and flexibility.
- Degradation Time: 18–24 months, depending on molecular weight and environmental conditions.
- Radial Force: 0.08–0.1 N/mm, comparable to nitinol stents for AVF applications.
- Manufacturing: Fused deposition modeling (FDM) 3D printing or laser-cut biaxial extrusion.
- Elastic Modulus: ~1.5 GPa for 3D-printed PLA; yield strength ~30.6 MPa.
- Coating Options: Drug-eluting variants (e.g., sirolimus, everolimus) for controlled release to prevent restenosis.